
Betty Vertin discusses her family's decision to take a significant risk in the hope of improving the health of three of their children.
I’m losing sleep. My husband, I, and three of our children made a big decision: a significant change.
I have three sons—Max, 17; Rowen,14; and Charlie, 12—all living with Duchenne muscular dystrophy (DMD). Duchenne is a rare, fatal form of muscular dystrophy. It causes muscle deterioration starting with the muscles in the hips and legs and eventually causing weakness in every muscle, including the lungs and heart. There is no cure. Life expectancy is mid-20s.
It’s been over a decade since our boys were diagnosed, and writing those sentences, their reality is brutal. Often, when I speak at an engagement and share those words, I see the beginning of tears in the eyes of many—my own included.
It’s a hard reality, but my family has hope.

A generation ago, there were no treatments for DMD, nothing being researched, no fundraising. The diagnosis was bleak, and boys died long before their mid-20s.
It was never that bleak for us. By the time our sons were diagnosed, specialists treating muscular dystrophy were using corticosteroids to manage some of the symptoms and were prolonging the lives of children diagnosed with DMD. When my sons were diagnosed in 2010, clinical trials were taking place, and scientists were taking steps that continued to improve the quality of life for people with the disease.
My oldest son was blessed to meet the criteria to participate in one of the studies. Shortly after his involvement, a sibling access program allowed my younger sons to participate in the study. The hope was that the drug they accessed during the trial would slow down the progression of the disease. Not knowing if it would work, we took a leap of faith when the boys enrolled in the study. Hope (and lack of options) pushed us to leap.
It was one of a minimal number of trial drugs available, and we felt blessed to participate.
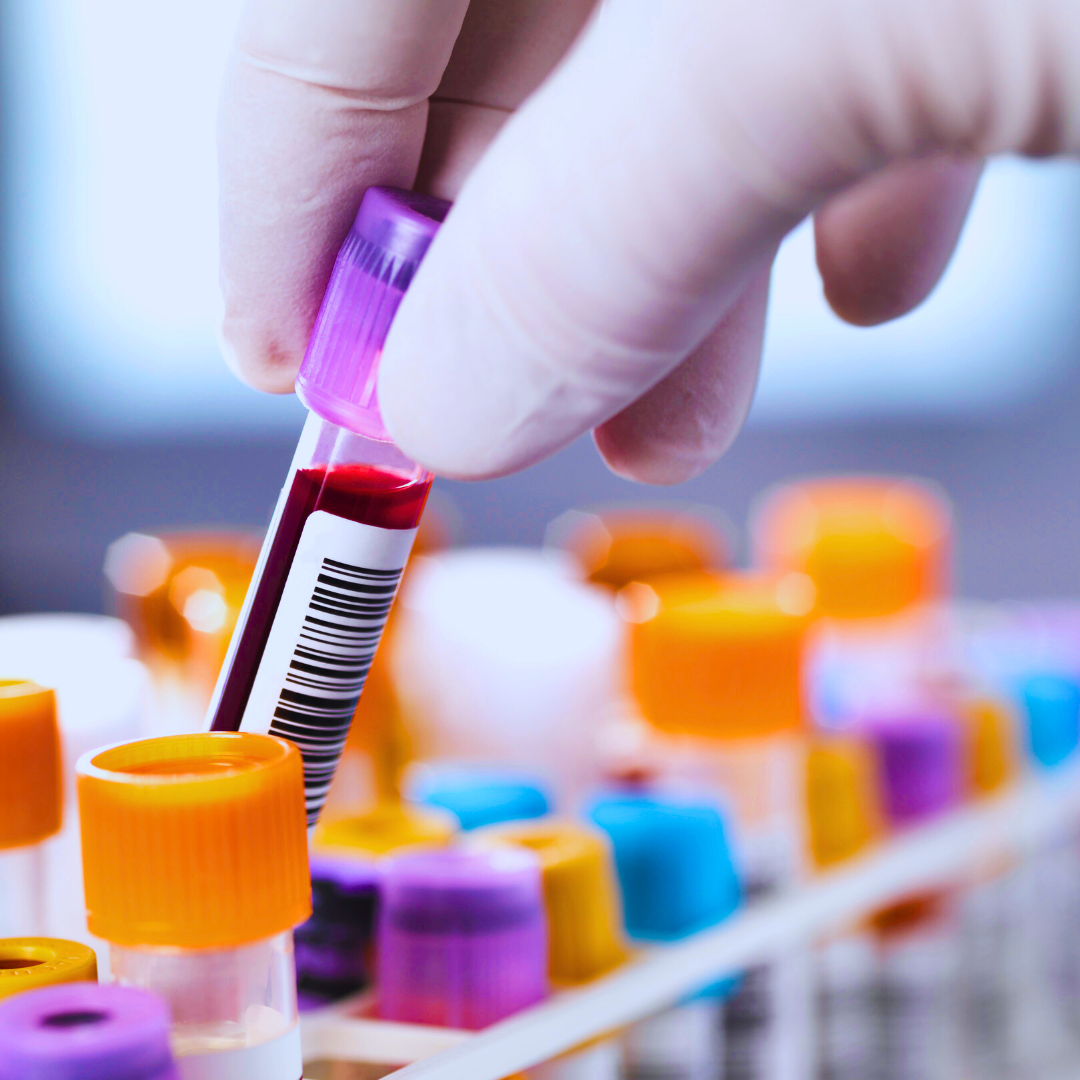
In the years that followed, we remained in the same trial. Our sons donated countless hours of their lives in the van traveling to and from appointments, consuming gallons of the juice we mixed with the medicine, and giving so many test tubes of blood and little cups of pee. They missed school and fun activities with their friends and family.
We knew the medicine was not a cure, but we hoped it would give us time with our children while a better treatment was developed. And our boys gave blood, sweat, and tears so that even if the treatment did not help them, someday, the things they were helping science learn would help someone else.
The better treatment we have been waiting for is here! Except, the very next moment, I lose my breath; I’m so anxious that I could be wrong. We don’t know for sure. It’s an unknown, but with the currently available information, it sounds like it could be the next move for our family.
To be eligible for the new treatment, which will come in a trial form again, our sons must stop using the first trial medicine and be off it for six months.
We have decided to stop the trial medicine they have been on for years. But we have no guarantee that our sons will pass the screening and have access to the new clinical trial drug. At this point, the new trial is not even open and may not be open in 6 months, or it may be canceled altogether. We don’t know. But we took the leap of faith anyway.
I can’t imagine making this decision for one of my other four healthy children. But being the mom of my three rare boys gives me the courage to do such things. And as a family of faith, we know that this leap may not lead us where we hope but to something or someone or someplace better. We trust it will work out how it is supposed to.

Copyright 2023 Betty Vertin
Images: Canva
About the Author

Betty Vertin
Betty Vertin is a Catholic wife, mother to 7, advocate, writer, and blogger living in Hastings, NE, with her family. Three of Betty’s children are boys living with Duchenne Muscular Dystrophy. Betty loves her Catholic faith and leans heavily on her parenting journey. She shares her family through social media, her blog Weathered-Storms.com and a column titled “Party of 9” for Muscular Dystrophy News.


.png?width=1806&height=731&name=CatholicMom_hcfm_logo1_pos_871c_2728c%20(002).png)
Comments